lewis structure of carbon|lewis diagram of co2 : iloilo Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to . ElCor Baliuag is on Facebook. Join Facebook to connect with ElCor Baliuag and others you may know. Facebook gives people the power to share and makes the.
lewis structure of carbon,Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to .lewis diagram of co21.2.2 Lewis Structures of Polyatomic Molecules or Ions. . Write a plausible .
To satisfy the octet of Carbon, one of the pairs of electrons on Oxygen must be .
Step 12. To attach a different atom to a carbon, first click the carbon with four . A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide). For the CO2 structure use the periodic table to find the total number of valence electrons for.
7.54M subscribers. Subscribed. 679. 113K views 3 years ago. This chemistry video explains how to draw the lewis structure of CO2 also known as Carbon Dioxide. It also .The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the .Lewis structure of carbon dioxide: This figure explains the bonding in a CO2 molecule. Each O atom starts out with six (red) electrons and C with four (black) electrons, .
chemistNATE. 252K subscribers. 871. 67K views 2 years ago. Carbon needs two double bonds, one to each of the two oxygens, to complete its octet. The atoms share electrons with .
Steps for Writing Lewis Structures. Example \(\PageIndex{2}\) 1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has 1 valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2.
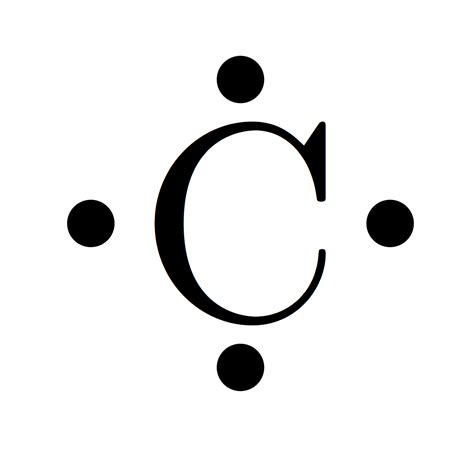
To satisfy the octet of Carbon, one of the pairs of electrons on Oxygen must be moved to create a double bond with Carbon. Therefore our Lewis Structure would look as it does below: The Hydrogen atoms are each filled up with their two electrons and both the Carbon and the Oxygen atoms' octets are filled.Lewis structure of a water molecule. Lewis structures – also called Lewis dot formulas, Lewis dot structures, . Hydrogen atoms bonded to carbon are not shown—they can be inferred by counting the number of bonds to a particular carbon atom—each carbon is assumed to have four bonds in total, so any bonds not shown are, by implication, to . A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below .Gilbert N. Lewis introduced a diagrammatic system to represent the valence shell electrons in an atom. It helps us a lot while doing chemical bonding. He used dots to represent the valence electrons. He used lines to indicate bond between two electrons. Here's an example of carbon, with its valence electrons using Lewis dot structure. Gilbert Lewis .Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial. Carbon needs two double bonds, one to each of the two oxygens, to complete its octet. The atoms *share* electrons with each other because they are both non-m.
1.2 Lewis Structure A Lewis structure shows the bonding between atoms as short lines (some books use pairs of dots) and non-bonding valence electrons as dots. . Write a plausible skeletal structure: Carbon atoms are always central, so the skeletal structure is: O — C — O. 3. Four electrons are used so far, and there are 16 – 4 = 12 .

A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound. A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.

A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound. A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.
This article contains detailed facts about 13 important factors of CO2 including CO2 lewis structure, bond angle, shape, etc. In the CO2 lewis structure, the shape of the molecule is linear. All the atoms of CO2 molecule lie in the same plane. The central atom c is sp hybridized here and there are two sigma bonds and two π bonds are . A step-by-step explanation of how to draw the Lewis Dot Structure for Carbon dioxide (CO2 ).For the Carbon dioxide structure we use the periodic table to fi.
A compound can have multiple Lewis Structures that contribute to the shape of the overall compound, so one Lewis structure of a compound may not necessarily be exactly what the compound looks like. . atoms. Usually, the central atom is already known as in the case with many organic compounds containing Carbon as the .The correct structure for carbonic acid. The central carbon has two bonds to an oxygen, and single bonds to two groups consisting of an oxygen and a hydrogen. Coming up with a Lewis structure requires a number of . Physical Properties of Diamond. has a very high melting point (almost 4000°C). Very strong carbon-carbon covalent bonds have to be broken throughout the structure before melting occurs. is very hard. This is again due to the need to break very strong covalent bonds operating in 3-dimensions. doesn't conduct electricity.
lewis structure of carbon lewis diagram of co2 Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, . The carbon monoxide is produced from the partial oxidation of carbon dioxide (CO2) or any other carbon-containing element. The Lewis structure, also called as electron dot structure, is a simplified method of representing the number of valence electrons present within an atom or a molecule. Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the .
lewis structure of carbon Here are the steps to draw a Lewis structure. The example is for the nitrate ion. A Lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.The diagram is also called a Lewis dot diagram, Lewis dot formula, or electron dot diagram.
lewis structure of carbon|lewis diagram of co2
PH0 · molecular structure of co2
PH1 · molecular orbital of co2
PH2 · mo diagram of co2
PH3 · lewis diagram of co2
PH4 · co2+ electron configuration
PH5 · co2 electronic structure
PH6 · Iba pa